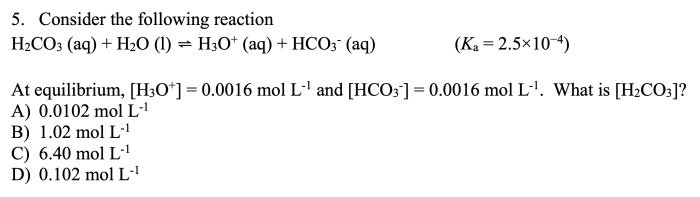
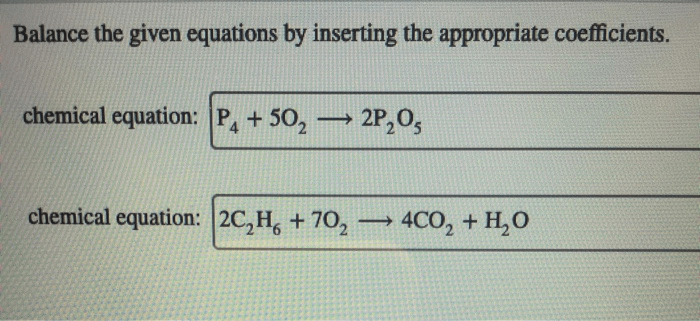
A container holds 6.4 moles of gas, a seemingly simple statement that opens the door to a fascinating exploration of gas behavior and its significance in various scientific disciplines. This article delves into the properties of gases, the Ideal Gas Law, gas stoichiometry, and gas mixtures, providing a comprehensive understanding of this fundamental aspect of chemistry.
The relationship between moles and volume of a gas is crucial in understanding gas behavior. The Ideal Gas Law establishes a connection between pressure, volume, temperature, and number of moles, enabling us to calculate the number of moles of gas present in a container.
Gas stoichiometry further extends this knowledge by allowing us to determine the number of moles of gas involved in chemical reactions.
Properties of Gases
Gases are characterized by their volume, pressure, temperature, and number of moles. The relationship between these properties is described by the Ideal Gas Law. The number of moles of gas in a container can be calculated using the formula:
Number of moles (n) = Volume (V) / Molar Volume (Vm)
Where molar volume (Vm) is the volume occupied by one mole of gas under standard conditions (0 °C and 1 atm) and is equal to 22.4 L/mol.
Examples:
- A container holds 6.4 moles of gas. What is the volume of the gas at standard conditions?
- A container contains 10 L of gas at a pressure of 2 atm and a temperature of 273 K. What is the number of moles of gas present?
Ideal Gas Law: A Container Holds 6.4 Moles Of Gas
The Ideal Gas Law combines the relationships between pressure (P), volume (V), temperature (T), and number of moles (n) of a gas. It is expressed as:
PV = nRT
Where R is the ideal gas constant, equal to 0.0821 L·atm/(mol·K).
Using the Ideal Gas Law to Calculate Number of Moles:
The Ideal Gas Law can be rearranged to solve for the number of moles:
Number of moles (n) = PV / RT
Example:, A container holds 6.4 moles of gas
A container contains 5 L of gas at a pressure of 3 atm and a temperature of 300 K. What is the number of moles of gas present?
Gas Stoichiometry
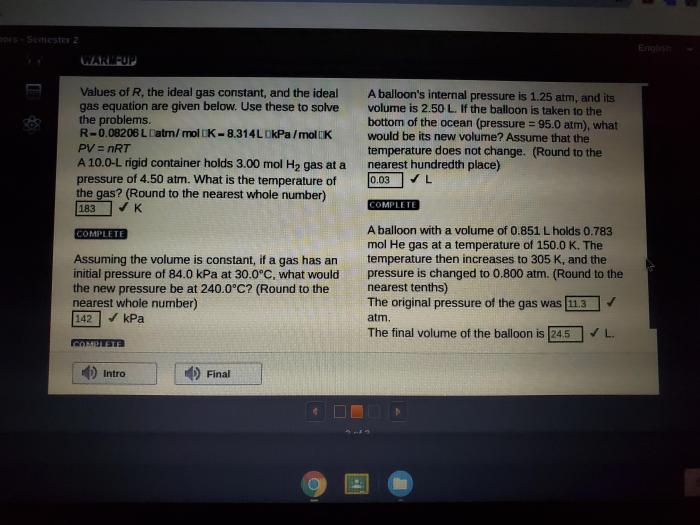
Gas stoichiometry deals with the quantitative relationships between reactants and products in gas-phase reactions. It uses the Ideal Gas Law to calculate the number of moles of gas involved in a reaction.
Using Gas Stoichiometry to Calculate Number of Moles:
To calculate the number of moles of gas involved in a reaction, the balanced chemical equation is used along with the Ideal Gas Law.
Example:, A container holds 6.4 moles of gas
In the reaction 2H2 + O2 → 2H2O, 10 L of hydrogen gas (H2) reacts completely with oxygen gas (O2) at standard conditions. What is the number of moles of water vapor (H2O) produced?
Gas Mixtures

Gas mixtures are composed of two or more gases. The partial pressure of each gas in a mixture is the pressure it would exert if it occupied the entire volume alone.
Dalton’s Law of Partial Pressures:
Dalton’s Law states that the total pressure of a gas mixture is equal to the sum of the partial pressures of the individual gases.
Example:, A container holds 6.4 moles of gas
A gas mixture contains 3 moles of nitrogen gas (N2) and 2 moles of oxygen gas (O2) in a 10 L container at a temperature of 298 K. What is the partial pressure of each gas and the total pressure of the mixture?
FAQ Compilation
What is the relationship between moles and volume of a gas?
The number of moles of gas is directly proportional to its volume at constant temperature and pressure.
How can I use the Ideal Gas Law to calculate the number of moles of gas?
Use the formula PV = nRT, where P is pressure, V is volume, n is number of moles, R is the gas constant, and T is temperature.
What is gas stoichiometry used for?
Gas stoichiometry helps determine the number of moles of gas involved in chemical reactions, enabling predictions about the products and reactants.