A carburizing flame has a white feather created by – Delving into the captivating realm of carburizing flames, we embark on an exploration of their unique characteristics, intricate chemical reactions, and diverse industrial applications. At the heart of these flames lies a mesmerizing phenomenon—the white feather—a radiant plume that captivates the eye and holds the key to understanding the transformative power of carburizing.
This flame’s distinctive appearance stems from the formation of carbon monoxide, which, upon reacting with oxygen, emits a brilliant white glow. The size and intensity of this feather vary depending on factors such as fuel composition, oxygen availability, and flame temperature, providing valuable insights into the carburizing process.
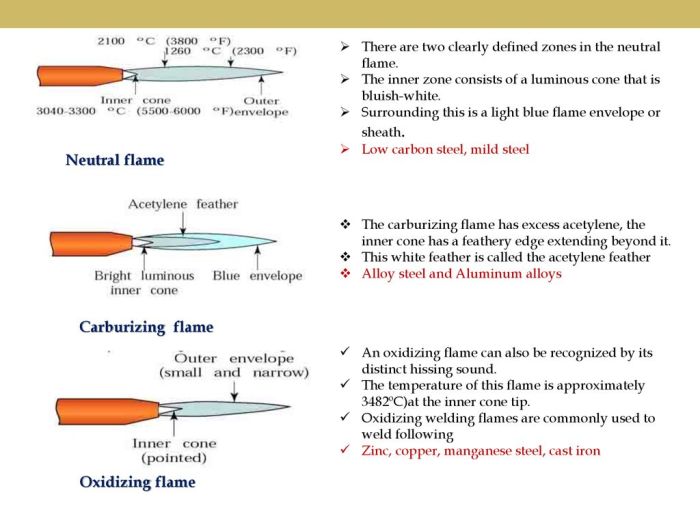
Flame Characteristics
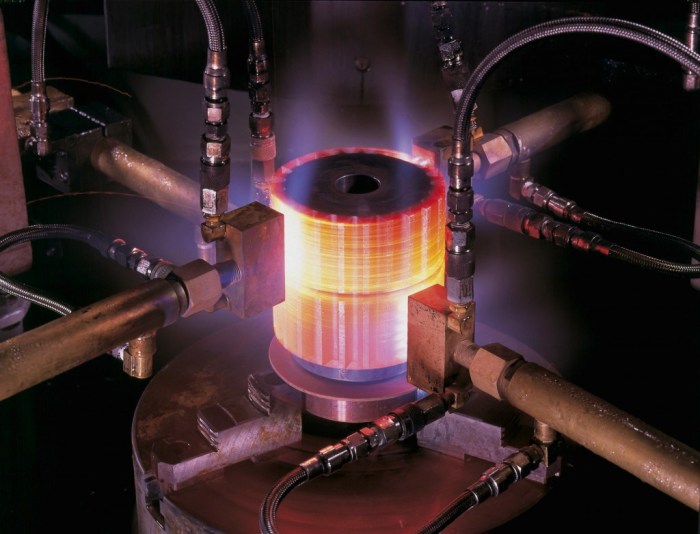
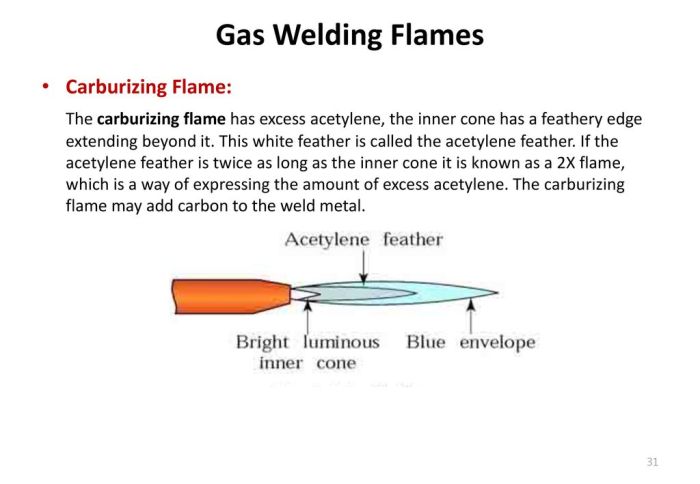
A carburizing flame is easily recognizable by its distinct appearance. The inner cone, where the fuel and air mix, emits a blue flame due to the combustion of the fuel. However, the outer envelope of the flame, where additional air is introduced, exhibits a characteristic white feather.
This white feather is created by the combustion of carbon monoxide (CO) that forms in the inner cone.
The white feather is composed primarily of carbon particles, which are incandescent due to the high temperature of the flame. The size and intensity of the white feather depend on several factors, including the fuel-to-air ratio, the rate of air entrainment, and the type of fuel used.
Chemical Reactions
The carburizing process occurs within the white feather of the flame. Carbon monoxide (CO) reacts with oxygen (O 2) to form carbon dioxide (CO 2), releasing heat in the process.
2CO + O 2→ 2CO 2+ heat
The carbon dioxide then reacts with the iron surface to form iron carbide (Fe 3C), which is a hard and wear-resistant material.
3CO 2+ Fe → Fe 3C + CO
Industrial Applications
Carburizing flames are used extensively in surface hardening processes. By exposing the surface of a metal to a carburizing flame, the carbon content of the surface is increased, resulting in a harder and more wear-resistant surface. This process is commonly used in the automotive, aerospace, and manufacturing industries.
Carburizing flames offer several advantages over other surface hardening methods. They are relatively inexpensive to operate, they can be used to treat large and complex parts, and they produce a consistent and uniform surface hardness.
However, carburizing flames also have some limitations. They can only be used on ferrous metals, and they can cause distortion of the part if not properly controlled.
Experimental Techniques
The carburizing depth of a flame can be measured using a variety of experimental techniques. One common method is to use a microhardness tester to measure the hardness of the surface at different depths.
Another method is to use a metallographic microscope to examine the microstructure of the surface. The carburizing depth can be determined by measuring the thickness of the carburized layer.
Historical Significance

Carburizing flames have been used for centuries to harden the surface of metals. The earliest known use of carburizing flames was in the production of swords and other weapons. In the 19th century, carburizing flames were used to harden the armor plates of warships.
Today, carburizing flames are used in a wide variety of industrial applications. They are essential for the production of many different types of products, including汽车零部件、航空航天部件和医疗器械。
User Queries: A Carburizing Flame Has A White Feather Created By
What causes the formation of the white feather in a carburizing flame?
The white feather is formed by the reaction of carbon monoxide with oxygen, producing a bright white glow.
What factors influence the size and intensity of the white feather?
The size and intensity of the white feather are influenced by fuel composition, oxygen availability, and flame temperature.
What are the industrial applications of carburizing flames?
Carburizing flames are used in surface hardening processes to increase the hardness and wear resistance of metal components.