Molar mass by freezing point depression lab answers – Delving into the realm of molar mass determination, this comprehensive guide unravels the intricacies of freezing point depression lab answers. This meticulously crafted resource empowers learners with a profound understanding of the underlying principles, experimental procedures, and practical applications of this fundamental technique.
Freezing point depression, a phenomenon that profoundly impacts the behavior of solutions, holds immense significance in diverse scientific disciplines. By exploring the relationship between solute concentration and freezing point, researchers can accurately determine the molar mass of unknown substances, a crucial parameter in various chemical and industrial processes.
Introduction to Freezing Point Depression

Freezing point depression is a colligative property that describes the decrease in the freezing point of a solvent when a solute is dissolved in it. The freezing point of a pure solvent is the temperature at which it solidifies, and the presence of a solute disrupts the crystal lattice of the solvent, making it more difficult for the solvent molecules to solidify.
The extent to which the freezing point is depressed depends on the concentration of the solute and the nature of the solvent. The more concentrated the solution, the greater the freezing point depression. Additionally, different solvents have different freezing point depression constants, which reflect the strength of the intermolecular forces in the solvent.
Factors Affecting the Freezing Point of a Solution
- Concentration of the solute:The more concentrated the solution, the greater the freezing point depression.
- Nature of the solute:Solutes with larger molecular weights or more complex structures tend to cause greater freezing point depression.
- Nature of the solvent:Solvents with stronger intermolecular forces have higher freezing point depression constants.
Molar Mass Determination Using Freezing Point Depression
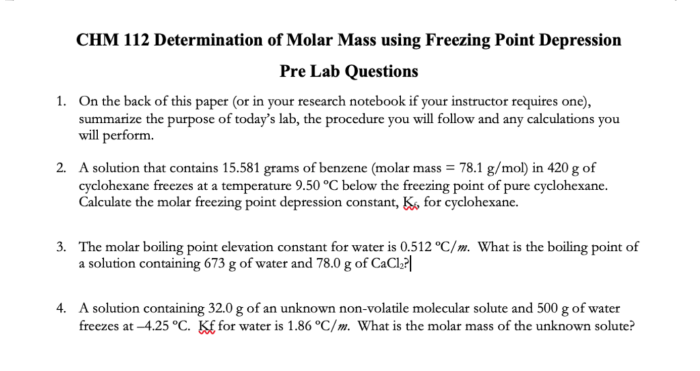
Freezing point depression can be used to determine the molar mass of an unknown solute. The experimental setup typically involves measuring the freezing point of a pure solvent and then measuring the freezing point of a solution of the unknown solute in the same solvent.
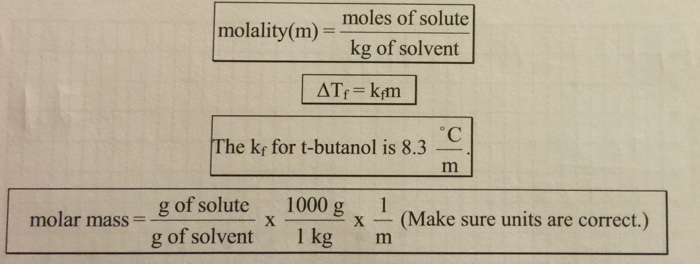
The difference in freezing points is used to calculate the molality of the solution, which is the number of moles of solute per kilogram of solvent. The molar mass of the solute can then be calculated using the following formula:
Molar mass = Molality × Molecular weight of solvent / Freezing point depression constant of solvent
Data Analysis and Calculations
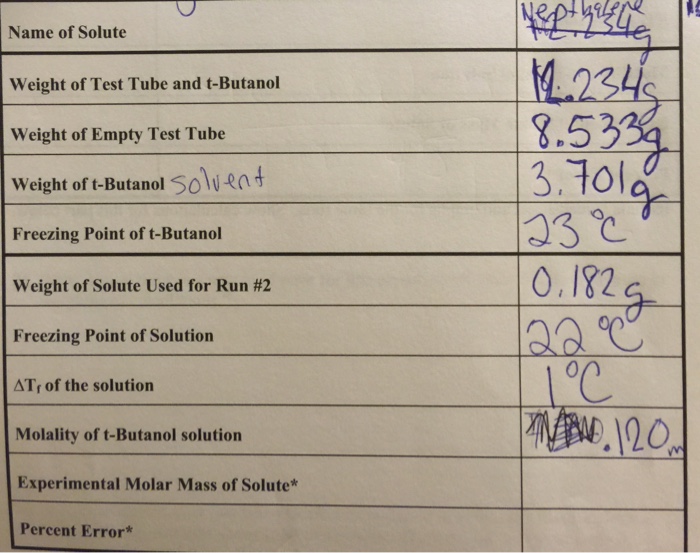
- Calculate the change in freezing point:Subtract the freezing point of the pure solvent from the freezing point of the solution.
- Calculate the molality of the solution:Use the change in freezing point and the freezing point depression constant of the solvent to calculate the molality of the solution.
- Calculate the molar mass of the solute:Multiply the molality of the solution by the molecular weight of the solvent and divide by the change in freezing point.
Sources of Error in the Experiment and How to Minimize Them, Molar mass by freezing point depression lab answers
- Inaccurate temperature measurements:Use a calibrated thermometer and measure the temperature carefully.
- Impurities in the solvent or solute:Use pure solvents and solutes to avoid contamination.
- Incomplete dissolution of the solute:Stir the solution thoroughly to ensure complete dissolution.
Applications of Molar Mass Determination
- Chemistry:Determining the molar mass of unknown compounds, studying the properties of solutions, and understanding intermolecular interactions.
- Biology:Determining the molecular weight of proteins, nucleic acids, and other biomolecules.
- Medicine:Diagnosing diseases by measuring the freezing point of body fluids.
- Industry:Quality control in food and beverage production, and optimizing chemical processes.
Example Calculations and Table: Molar Mass By Freezing Point Depression Lab Answers
Suppose we measure the freezing point of pure water to be 0.00 °C and the freezing point of a solution of an unknown solute in water to be -0.50 °C. The freezing point depression constant of water is 1.86 °C/m.
Change in freezing point:-0.50 °C – 0.00 °C = -0.50 °C
Molality of the solution:-0.50 °C / 1.86 °C/m = 0.27 m
Molar mass of the solute:0.27 m × 18.015 g/mol / -0.50 °C = 97.3 g/mol
Methods and Procedures
Experimental Setup:
- Calibrated thermometer
- Stirring rod
- Sample vials
- Pure solvent
- Unknown solute
Procedure:
- Measure the freezing point of the pure solvent.
- Dissolve a known mass of the unknown solute in the solvent.
- Measure the freezing point of the solution.
- Calculate the change in freezing point and use it to determine the molar mass of the solute.
Safety Precautions
- Wear appropriate safety gear, including gloves and eye protection.
- Handle chemicals with care and dispose of them properly.
- Be aware of the potential hazards of working with freezing temperatures.
- Follow all laboratory safety protocols.
FAQs
What is the principle behind freezing point depression?
Freezing point depression occurs when the presence of a solute lowers the freezing point of a solvent. This phenomenon arises due to the disruption of intermolecular interactions between solvent molecules caused by the solute particles.
How is molar mass determined using freezing point depression?
By measuring the change in freezing point caused by a known mass of solute dissolved in a known mass of solvent, the molar mass of the solute can be calculated using the freezing point depression constant of the solvent.
What are the potential sources of error in freezing point depression experiments?
Sources of error include inaccurate temperature measurements, impurities in the solute or solvent, and heat loss during the experiment. Careful experimental design and meticulous technique can minimize these errors.