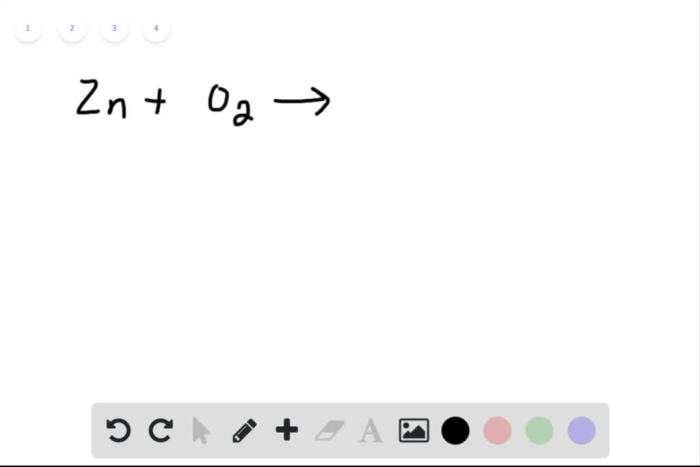
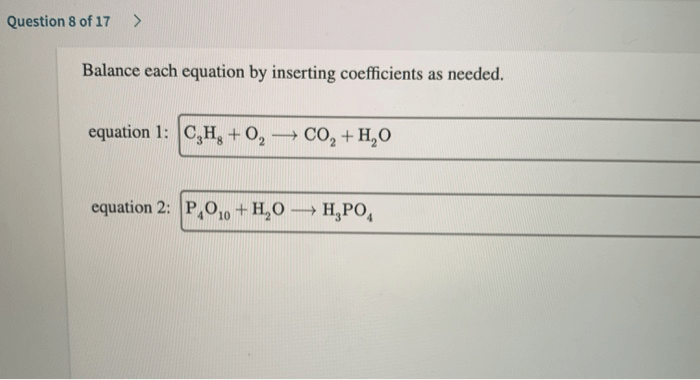
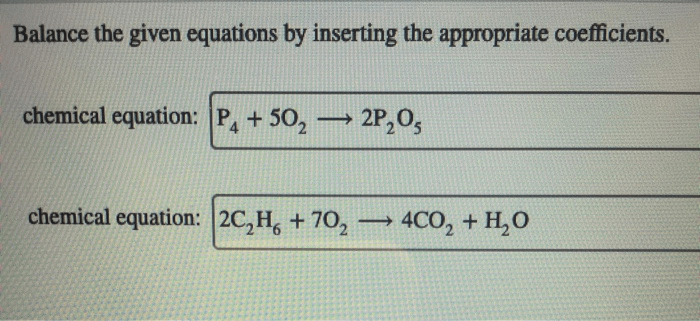
Balance the equation by inserting coefficients as needed – Balancing chemical equations by inserting coefficients is a crucial skill in chemistry. This comprehensive guide delves into the concept, methods, and applications of equation balancing, providing a thorough understanding of this fundamental aspect of chemical reactions.
The subsequent paragraphs explore the definition of balanced equations, the concept of coefficients, step-by-step procedures for balancing equations, and common methods such as oxidation-reduction and half-reaction methods. Additionally, examples of balanced equations, the use of fractions and decimals in coefficients, and the importance of maintaining the law of conservation of mass are discussed.
Balancing Chemical Equations
A balanced chemical equation is a chemical equation in which the number of atoms of each element is the same on both sides of the equation. This is important because the law of conservation of mass states that matter cannot be created or destroyed, so the number of atoms of each element must be the same before and after a reaction.
To balance a chemical equation, coefficients are used to adjust the number of atoms of each element on each side of the equation. Coefficients are numbers that are placed in front of each chemical formula in an equation. For example, the following equation is unbalanced:
“`
H2 + O2 → H2O
“`
This equation is unbalanced because there are 4 hydrogen atoms on the left side of the equation but only 2 hydrogen atoms on the right side of the equation. To balance this equation, we can add a coefficient of 2 in front of the H2O molecule:
“`
H2 + O2 → 2H2O
“`
This equation is now balanced because there are 4 hydrogen atoms on both sides of the equation.
Methods for Balancing Equations
There are a number of different methods that can be used to balance chemical equations. One common method is the oxidation-reduction method. This method is used to balance equations that involve redox reactions, which are reactions in which one or more atoms change their oxidation state.
Another common method is the half-reaction method. This method is used to balance equations that involve reactions in which water is produced or consumed.
Examples of Balanced Equations, Balance the equation by inserting coefficients as needed
The following are some examples of balanced chemical equations:
- 2H2 + O2 → 2H2O
- CH4 + 2O2 → CO2 + 2H2O
- 2Na + Cl2 → 2NaCl
Balancing Equations with Complex Coefficients
In some cases, it may be necessary to use fractions or decimals in coefficients to balance a chemical equation. For example, the following equation is unbalanced:
“`
Fe + 3O2 → Fe2O3
“`
This equation can be balanced by adding a coefficient of 2/3 in front of the Fe2O3 molecule:
“`
Fe + 3O2 → 2/3Fe2O3
“`
This equation is now balanced because there are 4 iron atoms and 6 oxygen atoms on both sides of the equation.
Applications of Balanced Equations
Balanced chemical equations are used in a variety of applications, including:
- Stoichiometry: Balanced chemical equations can be used to calculate the amount of reactants and products that are involved in a reaction.
- Predicting reaction outcomes: Balanced chemical equations can be used to predict the products of a reaction and the relative amounts of each product that will be formed.
Common Mistakes and Troubleshooting
There are a number of common mistakes that can be made when balancing chemical equations. Some of the most common mistakes include:
- Not checking to make sure that the equation is balanced for all of the elements.
- Using incorrect coefficients.
- Changing the chemical formulas of the reactants or products.
If you are having trouble balancing a chemical equation, there are a number of resources that can help you. You can find online tutorials, books, and software programs that can help you to balance equations.
FAQ Insights: Balance The Equation By Inserting Coefficients As Needed
What is the purpose of balancing chemical equations?
Balancing chemical equations ensures that the number of atoms of each element on the reactants’ side matches the number of atoms of the same element on the products’ side, adhering to the law of conservation of mass.
How do I know if an equation is balanced?
A balanced equation has the same number of atoms of each element on both the reactants’ and products’ sides.
What are the common methods for balancing chemical equations?
Common methods include the oxidation-reduction method, the half-reaction method, and the inspection method.