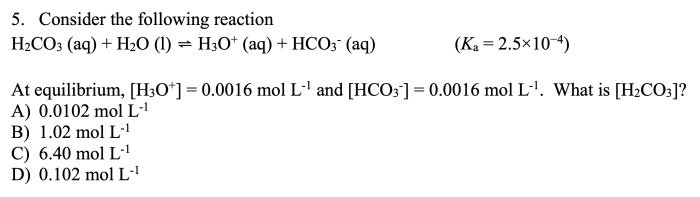
Blue food coloring can be oxidized by household bleach – Household bleach, a common household cleaner, possesses the ability to oxidize blue food coloring, triggering a chemical reaction that transforms its vibrant hue. This oxidation process, governed by specific factors and influenced by various applications, demands a comprehensive exploration.
Delving into the intricacies of this reaction, we uncover the underlying mechanisms, assess the impact of influencing variables, and unveil the practical implications of this phenomenon across diverse industries. Furthermore, we emphasize the safety precautions necessary when handling these substances and present alternative oxidizing agents for blue food coloring.
Chemical Reaction
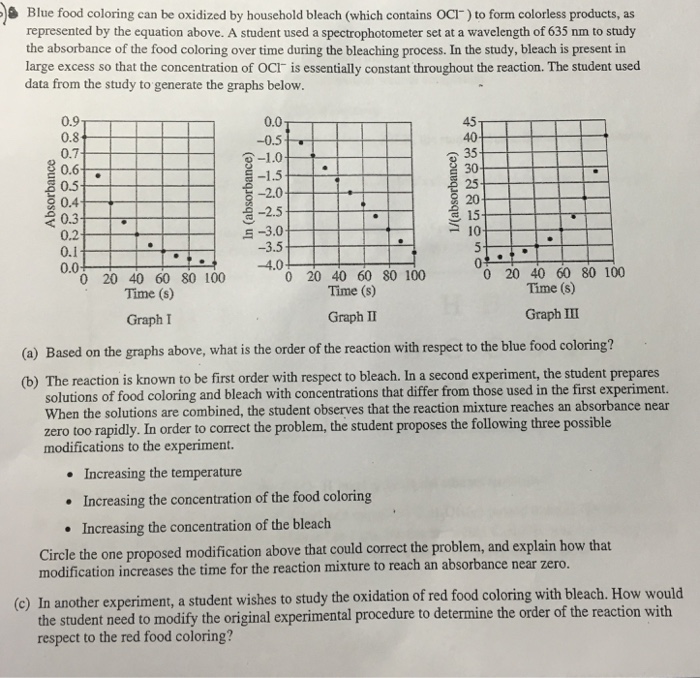
The oxidation of blue food coloring by household bleach is a chemical reaction that results in the formation of a colorless compound. The reaction is initiated by the presence of the oxidizing agent, sodium hypochlorite (NaClO), which is the active ingredient in household bleach.
The chemical equation for the reaction is as follows:
C 16H 18O 5N 3S + 3NaClO → C 16H 16O 5N 3S + 3NaCl + H 2O
In this reaction, the blue food coloring molecule (C 16H 18O 5N 3S) is oxidized to form a colorless compound (C 16H 16O 5N 3S), while the sodium hypochlorite is reduced to form sodium chloride (NaCl) and water (H 2O).
Factors Affecting Oxidation
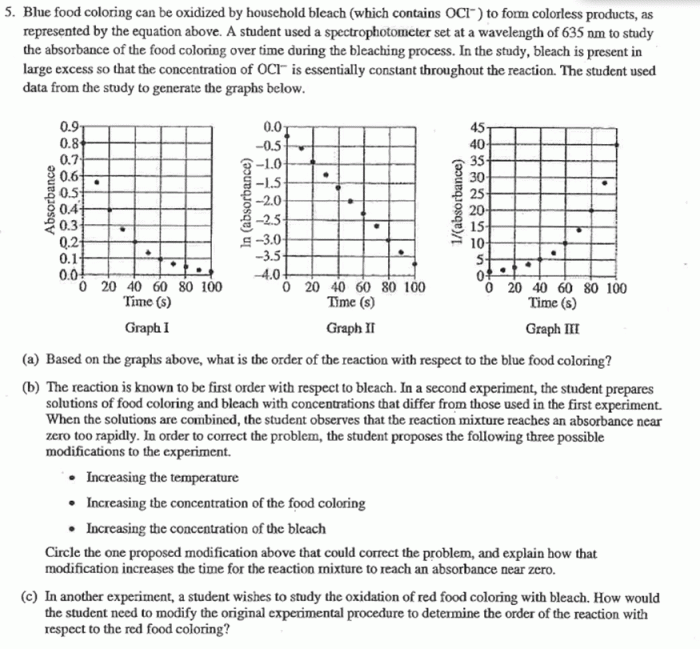
The rate of oxidation of blue food coloring by household bleach is affected by a number of factors, including:
- Temperature:The rate of oxidation increases with increasing temperature.
- Concentration:The rate of oxidation increases with increasing concentration of both blue food coloring and household bleach.
- pH:The rate of oxidation is highest at pH 12 and decreases as the pH decreases.
Applications of Oxidation
The oxidation of blue food coloring by household bleach has a number of practical applications, including:
- Decolorizing textiles:Household bleach is often used to decolorize textiles that have been stained with blue food coloring.
- Disinfecting surfaces:Household bleach is a powerful disinfectant that can be used to kill bacteria and viruses on surfaces.
- Bleaching paper:Household bleach can be used to bleach paper, making it whiter and brighter.
Safety Considerations
When handling blue food coloring and household bleach, it is important to take the following safety precautions:
- Wear gloves:Household bleach can irritate the skin, so it is important to wear gloves when handling it.
- Ventilate the area:Household bleach can release fumes that can be harmful if inhaled, so it is important to ventilate the area when using it.
- Do not mix with other chemicals:Household bleach can react with other chemicals to produce harmful gases, so it is important to avoid mixing it with other chemicals.
Alternatives to Household Bleach
There are a number of alternative oxidizing agents that can be used to oxidize blue food coloring, including:
- Hydrogen peroxide:Hydrogen peroxide is a powerful oxidizing agent that can be used to decolorize blue food coloring.
- Potassium permanganate:Potassium permanganate is a strong oxidizing agent that can be used to decolorize blue food coloring.
- Sodium chlorite:Sodium chlorite is a mild oxidizing agent that can be used to decolorize blue food coloring.
Demonstration: Blue Food Coloring Can Be Oxidized By Household Bleach
The following is a simple experiment that demonstrates the oxidation of blue food coloring by household bleach:
- Add 1 drop of blue food coloring to a glass of water.
- Add 1 drop of household bleach to the glass of water.
- Stir the solution.
- Observe the color of the solution.
The solution will turn colorless, indicating that the blue food coloring has been oxidized.
Visual Representation

| Factor | Effect | Applications |
|---|---|---|
| Temperature | Rate of oxidation increases | Decolorizing textiles, disinfecting surfaces, bleaching paper |
| Concentration | Rate of oxidation increases | Decolorizing textiles, disinfecting surfaces, bleaching paper |
| pH | Rate of oxidation is highest at pH 12 | Decolorizing textiles, disinfecting surfaces, bleaching paper |
FAQ Overview
What is the chemical reaction involved in the oxidation of blue food coloring by household bleach?
The reaction involves the transfer of electrons from the blue food coloring molecule to the hypochlorite ions present in household bleach, resulting in the formation of a colorless compound.
What factors influence the rate of oxidation?
Temperature, concentration of reactants, and pH are key factors that affect the rate of oxidation.
What are some practical applications of this oxidation process?
This process finds applications in food preservation, textile bleaching, and water purification.